PLASMOVITA PRF
The evolution of Platelet Rich Fibrin
Platelet concentrates have been utilized in medicine for over 3 decades owing to their ability to rapidly secrete growth factors. They have gained tremendous momentum as a regenerative agent derived from autologous sources capable of stimulating tissue regeneration in a number of medical fields.
Many years ago, it was proposed that by concentrating platelets utilizing a centrifugation device, autologous growth factors derived from blood could be collected from a platelet-rich plasma layer, and later utilized in surgical sites to promote local wound healing. Today, it has been well established that platelet concentrates act as a potent mitogen capable of:
1. Speeding the revascularization of tissues (angiogenesis)
2. Acting as a potent recruitment agent of various cells including stem cells (chemotaxis)
3. Inducing the prompt multiplication of various cell types found in the human body (proliferation)
Many years ago, it was proposed that by concentrating platelets utilizing a centrifugation device, autologous growth factors derived from blood could be collected from a platelet-rich plasma layer, and later utilized in surgical sites to promote local wound healing. Today, it has been well established that platelet concentrates act as a potent mitogen capable of:
1. Speeding the revascularization of tissues (angiogenesis)
2. Acting as a potent recruitment agent of various cells including stem cells (chemotaxis)
3. Inducing the prompt multiplication of various cell types found in the human body (proliferation)
Various systematic reviews from multiple fields of medicine have now demonstrated their ability to support the regeneration of a number of cell types and tissues. Below we review the evolution of platelet concentrates.
Platelet Rich Plasma (1990s)
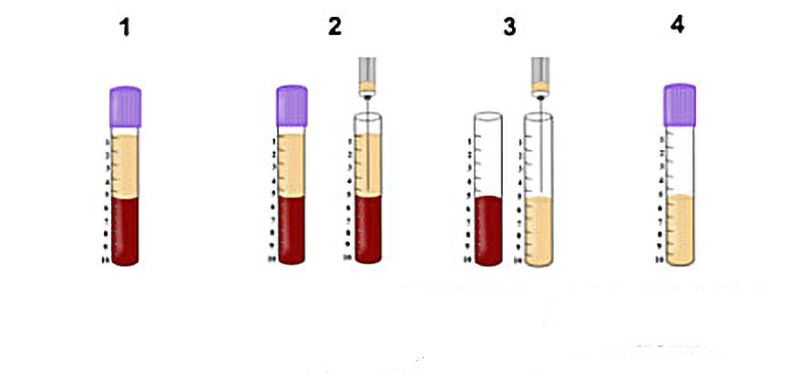
Work directed by Dr. Robert Marx in the 1990s led to the popular working name ‘platelet rich plasma’ (PRP). The goal of PRP was to collect the largest and highest concentrations of platelets/growth factors to be later utilized for regenerative purposes. The PRP protocol required extensive centrifugation time (typically over 30 minutes). During this process, the use of anticoagulants (namely bovine thrombin or calcium chloride) was absolutely necessary in order to prevent clotting owing to the lengthy centrifugation times. The final composition of PRP contained over 95% platelets, known cells responsible for the active secretion of growth factors involved in wound healing.
Two reported drawbacks of PRP have since been reported in the literature. First, centrifugation times were deemed long (>30 minutes) and not practical for a variety of surgical procedures that may routinely be performed in many clinical practices. Furthermore, it has since been revealed that clotting in general, is a necessary component of normal physiological wound healing. This limitation of using anti-coagulants prevented optimal and led to the development of platelet rich fibrin
Two reported drawbacks of PRP have since been reported in the literature. First, centrifugation times were deemed long (>30 minutes) and not practical for a variety of surgical procedures that may routinely be performed in many clinical practices. Furthermore, it has since been revealed that clotting in general, is a necessary component of normal physiological wound healing. This limitation of using anti-coagulants prevented optimal and led to the development of platelet rich fibrin
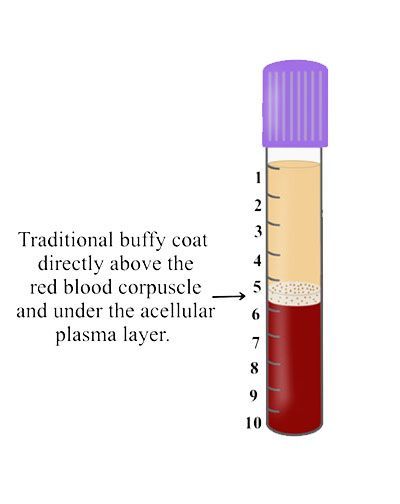
Figure: PRP was introduced with an ability to concentrate platelets within the buffy coat. This was effective because PRP utilized anti-coagulants whereby the majority of cells were found within the buffy coat. Various protocols and equipment have been introduced to isolate these cells but the concept remains that cells within the ‘buffy coat’ are isolated with use of anti-coagulants.
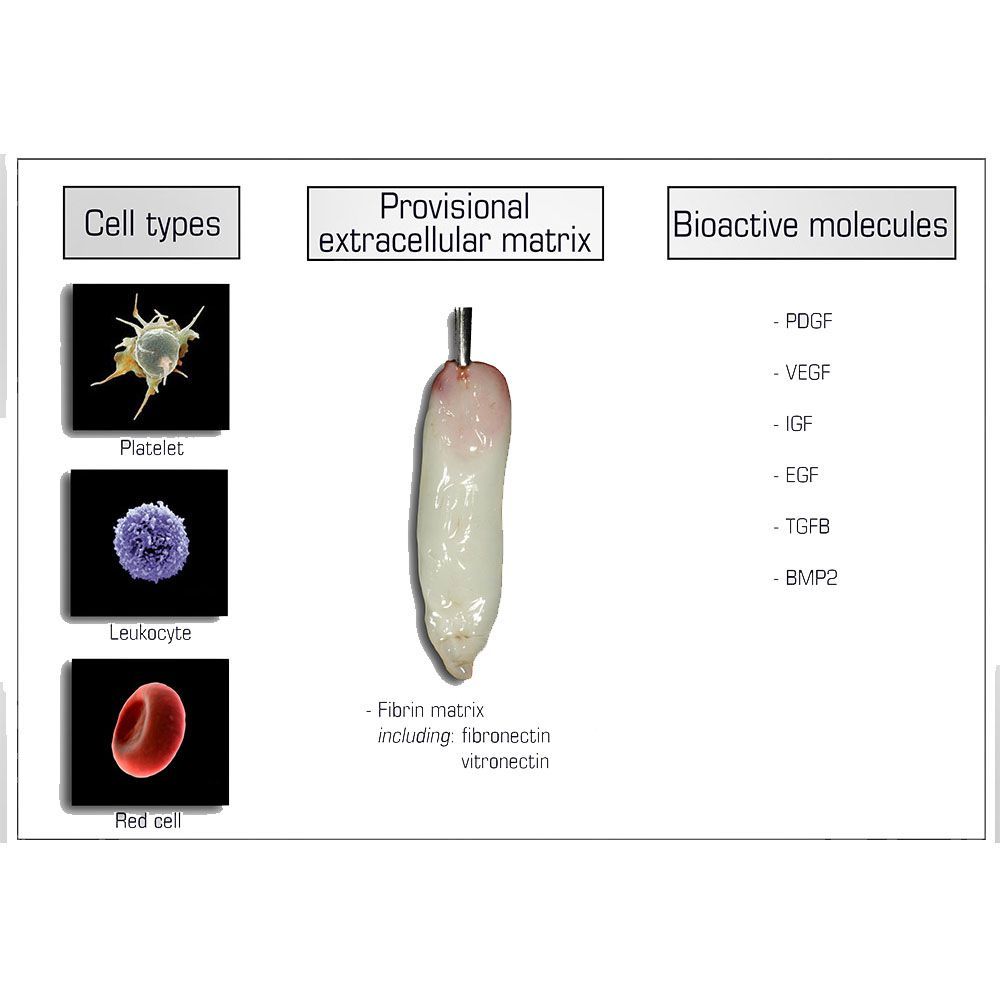
Leukocyte and Platelet Rich Fibrin:
L-PRF (2000-2010)
Owing to the drawback that the anticoagulants utilized in PRP prevented clotting, pioneering work led by dr. Joseph Choukroun and dr. David Dohan led to the development of platelet rich firin (PRF). The aim of PRF was to develop a second-generation platelet concentrate with anti-coagulant removal. Since anti-coagulants were removed, a much quicker working time was needed and the practioner absolutely required that centrifugation began shortly thereafter blood draw (otherwise blood would clot). Furthermore, high g-force centrifugation protocols were utilized in order to separate blood layers in attempt to separate blood layers prior to clotting.
The final spin cycle (2700 RPM for 12 minutes = 700g), resulted in a plasma layer composed of a fibrin clot with entrapment of platelets and leukocytes.
The final spin cycle (2700 RPM for 12 minutes = 700g), resulted in a plasma layer composed of a fibrin clot with entrapment of platelets and leukocytes.
Advanced and injectable Platelet Rich Fibrin:
A-PRF and i-PRF (2014-2018)
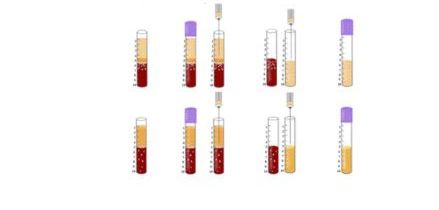
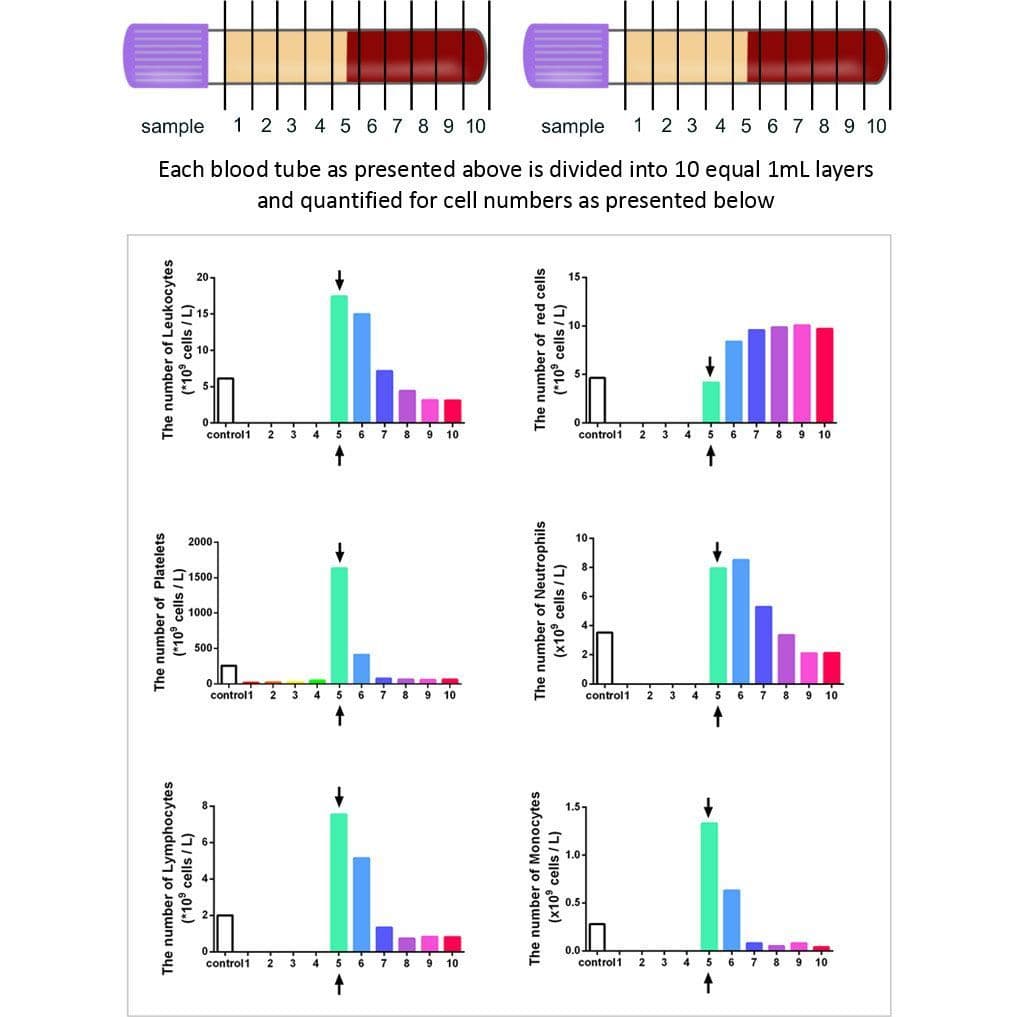
While much of the research performed in the late 2000s and early 2010s was dedicated to the clinical uses and indications of L-PRF, major discoveries were made several years following extensive clinical use of L-PRF. In 2014, an oral maxillofacial surgeon in Germany by the name of Dr. Shahram Ghanaati observed histologically that following centrifugation at high g-forces (~700g – utilized in L-PRF protocols), the majority of leukocytes and platelets were in fact located at the base of L-PRF clots or even worse, within the red corpuscle blood layer at the bottom of centrifugation tubes.
Pioneering research within his laboratory led to the development of an advanced platelet rich fibrin (A-PRF) whereby lower centrifugation speeds (~200g) led to a PRF members with more evenly distributed platelets. these newer protocols more favorably released a higher concentration of growth factors over a 10 day period when compared to PRP or L-PRF.
Pioneering research within his laboratory led to the development of an advanced platelet rich fibrin (A-PRF) whereby lower centrifugation speeds (~200g) led to a PRF members with more evenly distributed platelets. these newer protocols more favorably released a higher concentration of growth factors over a 10 day period when compared to PRP or L-PRF.
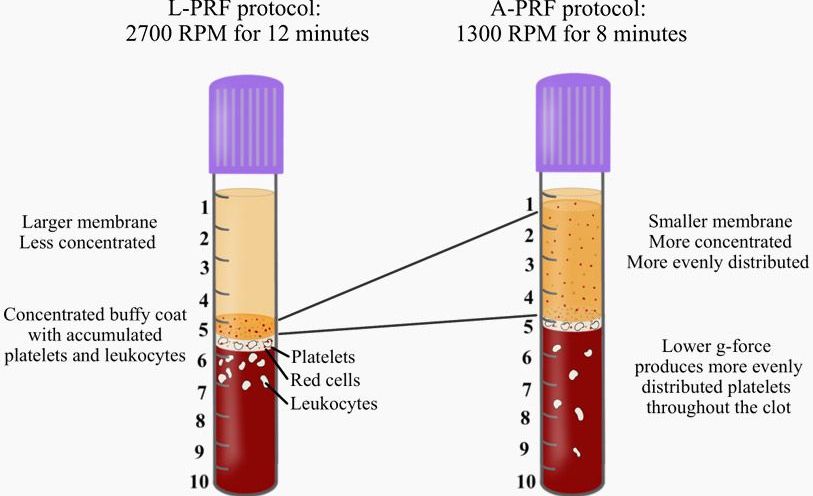
Comparing cell distribution in L-PRF and A-PRF protocols
Horizontal Centrifugation: Plasmovita PRF
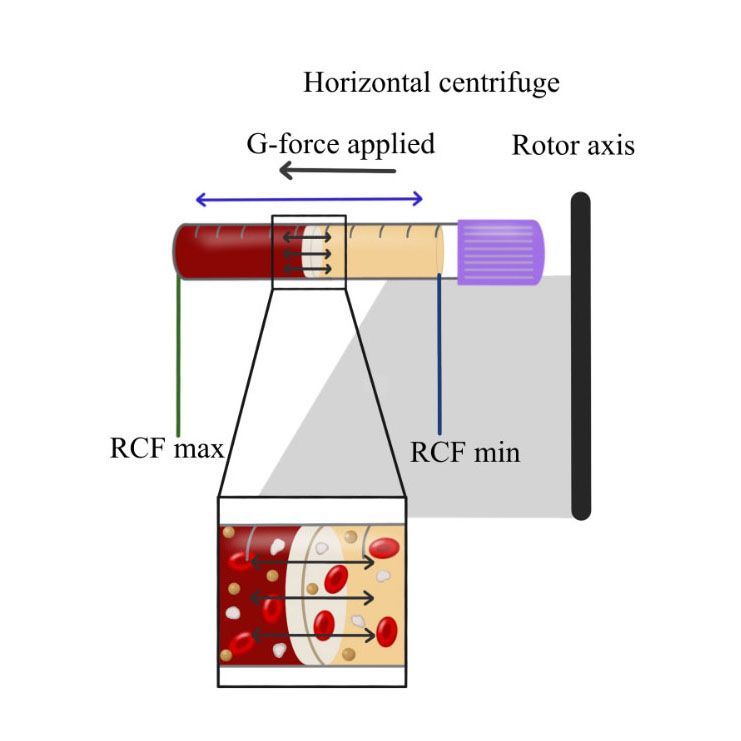
Very recently, a series of studies has shown that horizontal centrifugation of PRF is capable of producing significantly greater concentrations of platelets and leukocytes when compared to the currently available fixed-angle centrifugation devices most commonly utilized to produce L-PRF or A-PRF.
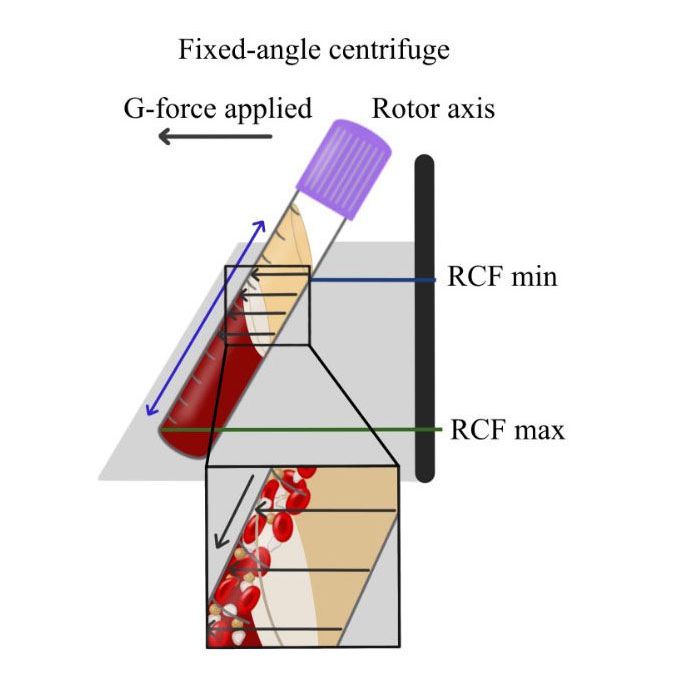
It was reported that one of the major disadvantages of fixed-angle centrifugation is that during the spin cycles, cells are typically driven along the back wall of centrifugation tubes at high g-forces. This exposes cells to high compressive forces against the back wall and must thereafter travel either up or down the inclined centrifugation tube based on cell density differences. Since red blood cells are larger and heavier than platelets and leukocytes, they typically travel downwards, whereas lighter platelets travel towards the top of the tube where PRF is collected. Noteworthy however, it was shown that on a fixed angle centrifuge, cells accumulate at the back walls of centrifugation tubes and the larger red blood cells entrap the smaller platelets/leukocytes below them and drag them into the red corpuscle layer. By utilizing a fixed-angle centrifuge, it is not possible to maximize platelet and leukocyte numbers as a result of fixed-angle centrifugation.
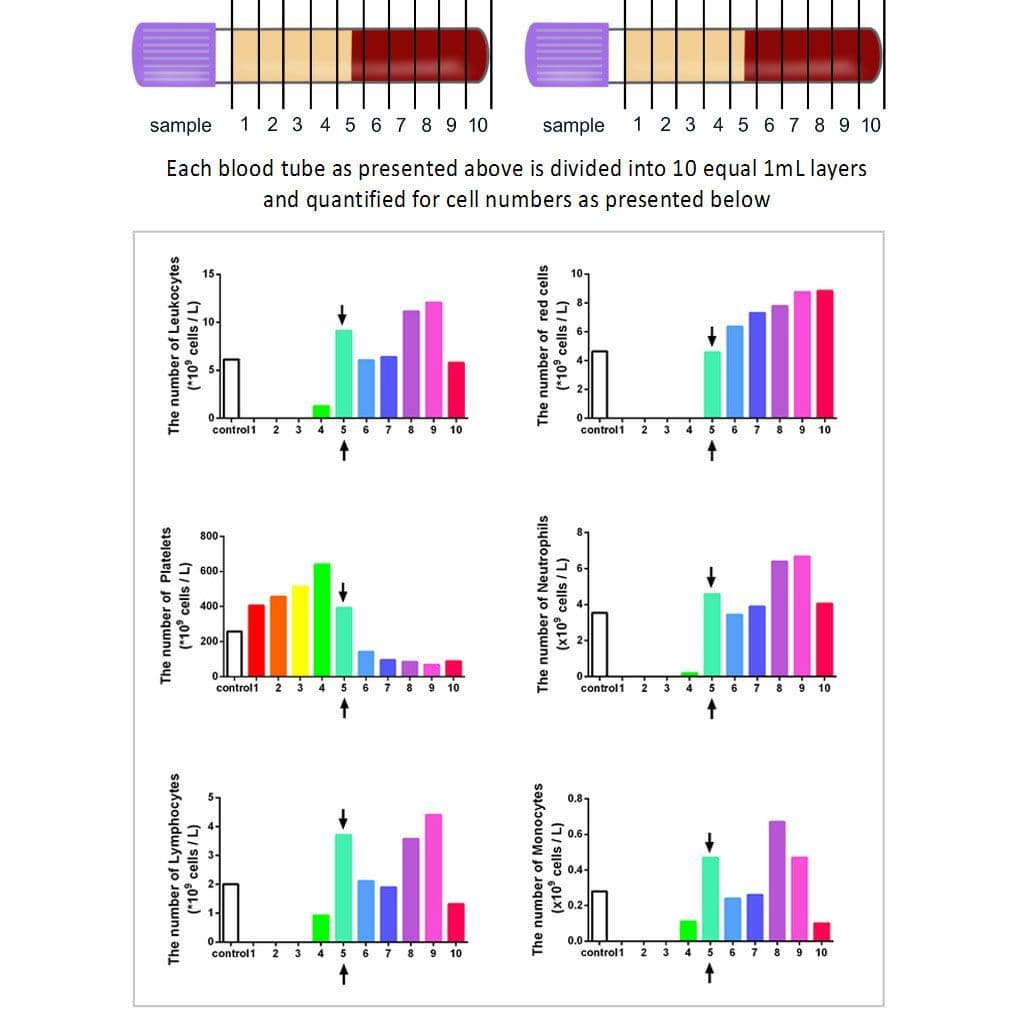
By utilizing a horizontal swing-out bucket centrifugation system, it becomes possible to fully separate cells and blood layers based on their density without necessitating cells to accumulate/damage on the back walls of centrifugation tubes. By utilizing horizontal centrifugation, it therefore becomes possible to isolate a higher number and concentration of platelets, leukocytes and monocytes when compared to either the L-PRF or A-PRF protocols.
It was reported that one of the major disadvantages of fixed-angle centrifugation is that during the spin cycles, cells are typically driven along the back wall of centrifugation tubes at high g-forces. This exposes cells to high compressive forces against the back wall and must thereafter travel either up or down the inclined centrifugation tube based on cell density differences. Since red blood cells are larger and heavier than platelets and leukocytes, they typically travel downwards, whereas lighter platelets travel towards the top of the tube where PRF is collected. Noteworthy however, it was shown that on a fixed angle centrifuge, cells accumulate at the back walls of centrifugation tubes and the larger red blood cells entrap the smaller platelets/leukocytes below them and drag them into the red corpuscle layer. By utilizing a fixed-angle centrifuge, it is not possible to maximize platelet and leukocyte numbers as a result of fixed-angle centrifugation.
By utilizing a horizontal swing-out bucket centrifugation system, it becomes possible to fully separate cells and blood layers based on their density without necessitating cells to accumulate/damage on the back walls of centrifugation tubes. By utilizing horizontal centrifugation, it therefore becomes possible to isolate a higher number and concentration of platelets, leukocytes and monocytes when compared to either the L-PRF or A-PRF protocols.

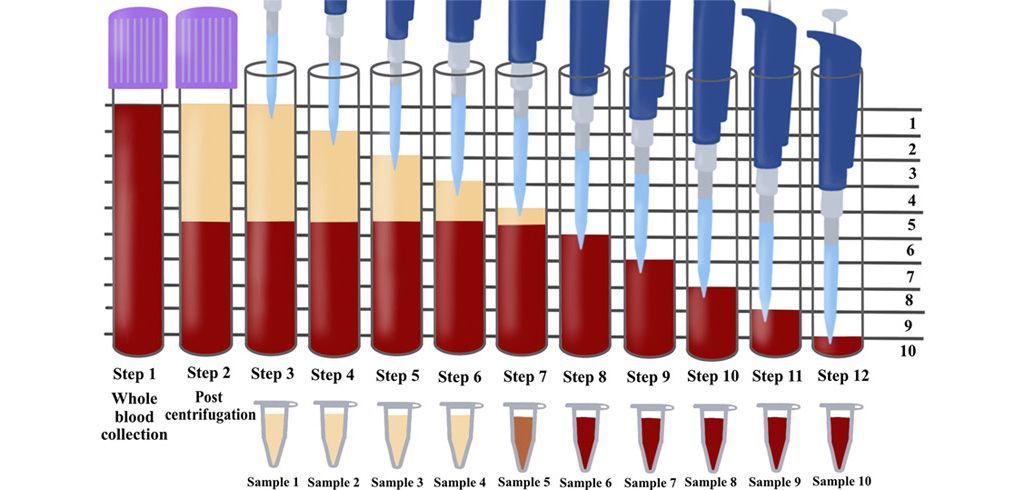
Figure: The concentration of cell types in each layer from 1mL down to the 10th mL sample utilizing the solid-PRF horizontal centrifugation protocol (700g for 8 minutes). Notice that most of the platelets as well as white blood cells are now more evenly distributed throughout the upper plasma layer. This is the first protocol generated for the production of PRF actually demonstrating an increase of white blood cells distributed throughout the upper PRF-layers.
Research
References
Anfossi G, Trovati M, Mularoni E, Massucco P, Calcamuggi G, Emanuelli G. 1989. Influence of propranolol on platelet aggregation and thromboxane b2 production from platelet-rich plasma and whole blood. Prostaglandins, leukotrienes, and essential fatty acids. 36(1):1-7.
Cai YZ, Zhang C, Lin XJ. 2015. Efficacy of platelet-rich plasma in arthroscopic repair of full-thickness rotator cuff tears: A meta-analysis. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons [et al]. 24(12):1852-1859.
Choukroun J, Ghanaati S. 2018. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (prf) concentrates advances patients’ own inflammatory cells, platelets and growth factors: The first introduction to the low speed centrifugation concept. European journal of trauma and emergency surgery : official publication of the European Trauma Society. 44(1):87-95.
Chow TW, McIntire LV, Peterson DM. 1983. Importance of plasma fibronectin in determining pfp and prp clot mechanical properties. Thrombosis research. 29(2):243-248.
Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. 2010. Three-dimensional architecture and cell composition of a choukroun’s platelet-rich fibrin clot and membrane. Journal of periodontology. 81(4):546-555.
Ehrenfest DMD, Rasmusson L, Albrektsson TJTib. 2009. Classification of platelet concentrates: From pure platelet-rich plasma (p-prp) to leucocyte-and platelet-rich fibrin (l-prf). 27(3):158-167.
El Bagdadi K, Kubesch A, Yu X, Al-Maawi S, Orlowska A, Dias A, Booms P, Dohle E, Sader R, Kirkpatrick CJ et al. 2017. Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (prf)-based matrices: A proof of concept of lscc (low speed centrifugation concept). European journal of trauma and emergency surgery : official publication of the European Trauma Society.
Fijnheer R, Pietersz RN, de Korte D, Gouwerok CW, Dekker WJ, Reesink HW, Roos D. 1990. Platelet activation during preparation of platelet concentrates: A comparison of the platelet-rich plasma and the buffy coat methods. Transfusion. 30(7):634-638.
Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J. 2017. Optimized platelet-rich fibrin with the low-speed concept: Growth factor release, bio compatibility, and cellular response. Journal of periodontology. 88(1):112-121.
Ghanaati S, Al-Maawi S, Herrera-Vizcaino C, Alves GG, Calasans-Maia MD, Sader R, Kirkpatrick CJ, Choukroun J, Bonig H, Mourao C. 2018. A proof of the low speed centrifugation concept in rodents: New perspectives for in vivo research. Tissue engineering Part C, Methods. 24(11):659-670.
Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, Landes C, Sader R, Kirkpatrick C, Choukroun J. 2014. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. The Journal of oral implantology. 40(6):679-689.
Jameson C. 2007. Autologous platelet concentrate for the production of platelet gel. Lab Med. 38:39-42.
Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ. 2016a. Comparative release of growth factors from prp, prf, and advanced-prf. Clinical oral investigations.
Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ. 2016b. Comparative release of growth factors from prp, prf, and advanced-prf. Clinical oral investigations. 20(9):2353-2360.
Kubesch A, Barbeck M, Al-Maawi S, Orlowska A, Booms PF, Sader RA, Miron RJ, Kirkpatrick CJ, Choukroun J, Ghanaati S. 2018. A low-speed centrifugation concept leads to cell accumulation and vascularization of solid platelet-rich fibrin: An experimental study in vivo. Platelets.1-12.
Marx RE. 2004. Platelet-rich plasma: Evidence to support its use. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 62(4):489-496.
Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. 1998. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 85(6):638-646.
Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. 2016. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review.
Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 32(3):495-505.
Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, Choukroun J. 2017a. Injectable platelet rich fibrin (i-prf): Opportunities in regenerative dentistry? Clinical oral investigations.
Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, Fujioka-Kobayashi M, Bishara M, Zhang Y, Wang HL et al. 2017b. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clinical oral investigations. 21(6):1913-1927.
Singh B, Goldberg LJ. 2016. Autologous platelet-rich plasma for the treatment of pattern hair loss. American journal of clinical dermatology.
Wang X, Zhang Y, Choukroun J, Ghanaati S, Miron RJ. 2017. Behavior of gingival fibroblasts on titanium implant surfaces in combination with either injectable-prf or prp. International journal of molecular sciences. 18(2).
Cai YZ, Zhang C, Lin XJ. 2015. Efficacy of platelet-rich plasma in arthroscopic repair of full-thickness rotator cuff tears: A meta-analysis. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons [et al]. 24(12):1852-1859.
Choukroun J, Ghanaati S. 2018. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (prf) concentrates advances patients’ own inflammatory cells, platelets and growth factors: The first introduction to the low speed centrifugation concept. European journal of trauma and emergency surgery : official publication of the European Trauma Society. 44(1):87-95.
Chow TW, McIntire LV, Peterson DM. 1983. Importance of plasma fibronectin in determining pfp and prp clot mechanical properties. Thrombosis research. 29(2):243-248.
Delaini F, Poggi A, Donati MB. 1982. Enhanced affinity for arachidonic acid in platelet-rich plasma from rats with adriamycin-induced nephrotic syndrome. Thrombosis and haemostasis. 48(3):260-262.
Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. 2010. Three-dimensional architecture and cell composition of a choukroun’s platelet-rich fibrin clot and membrane. Journal of periodontology. 81(4):546-555.
Ehrenfest DMD, Rasmusson L, Albrektsson TJTib. 2009. Classification of platelet concentrates: From pure platelet-rich plasma (p-prp) to leucocyte-and platelet-rich fibrin (l-prf). 27(3):158-167.
El Bagdadi K, Kubesch A, Yu X, Al-Maawi S, Orlowska A, Dias A, Booms P, Dohle E, Sader R, Kirkpatrick CJ et al. 2017. Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (prf)-based matrices: A proof of concept of lscc (low speed centrifugation concept). European journal of trauma and emergency surgery : official publication of the European Trauma Society.
Fijnheer R, Pietersz RN, de Korte D, Gouwerok CW, Dekker WJ, Reesink HW, Roos D. 1990. Platelet activation during preparation of platelet concentrates: A comparison of the platelet-rich plasma and the buffy coat methods. Transfusion. 30(7):634-638.
Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J. 2017. Optimized platelet-rich fibrin with the low-speed concept: Growth factor release, bio compatibility, and cellular response. Journal of periodontology. 88(1):112-121.
Ghanaati S, Al-Maawi S, Herrera-Vizcaino C, Alves GG, Calasans-Maia MD, Sader R, Kirkpatrick CJ, Choukroun J, Bonig H, Mourao C. 2018. A proof of the low speed centrifugation concept in rodents: New perspectives for in vivo research. Tissue engineering Part C, Methods. 24(11):659-670.
Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, Landes C, Sader R, Kirkpatrick C, Choukroun J. 2014. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. The Journal of oral implantology. 40(6):679-689.
Jameson C. 2007. Autologous platelet concentrate for the production of platelet gel. Lab Med. 38:39-42.
Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ. 2016a. Comparative release of growth factors from prp, prf, and advanced-prf. Clinical oral investigations.
Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ. 2016b. Comparative release of growth factors from prp, prf, and advanced-prf. Clinical oral investigations. 20(9):2353-2360.
Kubesch A, Barbeck M, Al-Maawi S, Orlowska A, Booms PF, Sader RA, Miron RJ, Kirkpatrick CJ, Choukroun J, Ghanaati S. 2018. A low-speed centrifugation concept leads to cell accumulation and vascularization of solid platelet-rich fibrin: An experimental study in vivo. Platelets.1-12.
Marx RE. 2004. Platelet-rich plasma: Evidence to support its use. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 62(4):489-496.
Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. 1998. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 85(6):638-646.
Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. 2016. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review.
Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 32(3):495-505.
Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, Choukroun J. 2017a. Injectable platelet rich fibrin (i-prf): Opportunities in regenerative dentistry? Clinical oral investigations.
Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, Fujioka-Kobayashi M, Bishara M, Zhang Y, Wang HL et al. 2017b. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clinical oral investigations. 21(6):1913-1927.
Singh B, Goldberg LJ. 2016. Autologous platelet-rich plasma for the treatment of pattern hair loss. American journal of clinical dermatology.
Wang X, Zhang Y, Choukroun J, Ghanaati S, Miron RJ. 2017. Behavior of gingival fibroblasts on titanium implant surfaces in combination with either injectable-prf or prp. International journal of molecular sciences. 18(2).